usp endotoxin limits|endotoxin limits for parenteral drugs : Manila USP's revision of the standard for Bacterial Endotoxins has been approved by the Pharmacopeial Discussion Group (PDG) and will be incorporated with the second supplement within USP 35- NF 30. Interested applicants for the caregiver position can now apply at www.poea.gov.ph. The agency said they will choose the 500 qualified applicants by lottery. Earlier, POEA announced that the State of Israel’s Ministry of Interior (MOI) has allowed the entry and re-entry of foreign workers in the caregiving sector. Even those who are .
usp endotoxin limits,USP's revision of the standard for Bacterial Endotoxins has been approved by the Pharmacopeial Discussion Group (PDG) and will be incorporated with the second supplement within USP 35- NF 30.ilution of a specimen at which the endotoxin l. on:MVD = (endotoxin limit ́ .
For radiopharmaceutical products not administered intrathecally the endotoxin limit is calculated as 175/ V, where V is the maximum recommended dose in mL. For .
usp endotoxin limits endotoxin limits for parenteral drugsilution of a specimen at which the endotoxin l. on:MVD = (endotoxin limit ́ concentration of Sample Solu-tion)/(l)Endotoxin Limit—The endotoxin limit for parenteral drugs, .
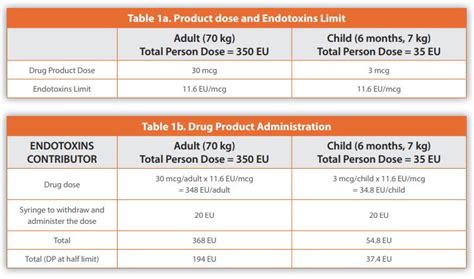
Water for Injection, Sterile Water for Injection and Sterile Water for Irrigation have an allowable endotoxin limit of 0.25 Endotoxin Units (EU)/ml. (EU=Unit of measurement for . The lower the dose, the higher the limit per unit dose. Think about it this way: If the dose is 1 mg/kg/hr, the endotoxin limit is (5 EU/kg/hr) ÷ (1 mg/kg/hr) = 5 EU/mg; If the dose is 10 mg/kg/hr, the .
Endotoxin limit: the endotoxin limit for active substances administered parenterally, defined on the basis of dose, is equal to: = threshold pyrogenic dose of endotoxin per .The Center for Devices and Radiological Health (CDRH) has adopted the USP Endotoxin Reference Standard and limits for medical device extracts expressed in EU/mL.
The purpose of this information chapter is to provide additional background information and guidance for the performance and proper application of the compendial bacterial .Endotoxin Limit The endotoxin limit for medicinal products administered parenterally, is defined on the basis of dose. Calculate the endotoxin limit as follows: Result = K /M K .K*N/V is used to convert the limit from EU/device to EU/mL, with K being the endotoxin limit, N the number of devices, and V the total extract volume used. This formula considers .

Endotoxin limit = K /M = 100 EU/ m2 / 30 mg/m2 = 3.33 EU/mg Medical Devices USP chapter <161>4 set generic endotoxin limits of 20 EU/device for most devices labeled as non-pyrogenic and 2.15 EU/device for devices that contact the cerebrospinal fluid (CSF). As an aqueous solution is required for the BET,endotoxin limits for parenteral drugsEndotoxin limit = K /M = 100 EU/ m2 / 30 mg/m2 = 3.33 EU/mg Medical Devices USP chapter <161>4 set generic endotoxin limits of 20 EU/device for most devices labeled as non-pyrogenic and 2.15 EU/device for devices that contact the cerebrospinal fluid (CSF). As an aqueous solution is required for the BET,After the withdrawal of FDA’s 1987 guideline on the LAL test, there were gaps in the regulatory documents regarding certain aspects of endotoxin testing like RSE/CSE standardization. On 01 December 2019, USP therefore published General Chapter <1085> Guidelines on the Endotoxins Test. This new chapter aims to “provide additional .13. Mar. According to USP Chapter 161, “Transfusion and Infusion Assemblies and Similar Medical Devices,” endotoxin limits for medical devices are as follows: For devices that come into direct or indirect contact with the cardiovascular system and lymphatic system, the endotoxin limit is 0.5 EU/mL or 20 EU/device.2.1.2 The Endotoxin Reference Standard should be calibrated to the current WHO (World Health Organization) International Standard for Endotoxin. 2.2. Acceptance Criteria The evaluated texts did not contain acceptance criteria. Endotoxin limits should be specified in the application dossier unless otherwise specified in an individual monograph. 3.The pre-administration exposure duration and temperature limits specified in the following low-risk, medium-risk, and high-risk level sections apply in the absence of direct testing results or appropriate information sources that justify different limits for specific CSPs. . the CSP must not exceed the amount of USP Endotoxin Units (EU per . The USP General Chapter Bacterial Endotoxins Test recommended maximum endotoxin exposure is NMT 5 EU/kg (interpreted as within 1 hour) for most drugs based on an average patient weight of 70 kg. . Question 2: Is it acceptable to omit bacterial endotoxin limits in the proposed specification for a topical ophthalmic drug product.The endotoxin units set by United States Pharmacopoeia (USP), and the techniques specified by USP for endotoxin testing are described. Endotoxin limits for preclinical research animal models were derived based on the threshold pyrogenic human dose of 5 E.U. per kg. The limits calculated would act as a guideline for endotoxin limits in .Pyrogen and Endotoxins Testing: Questions and Answers June 2012. This guidance provides recommendations for biological product, drug, and device firms on FDA’s current thinking concerning the .
According to the pharmacopoeia (e.g. USP or Ph.Eur.), Purified Water does not have to be tested for endotoxins. The endotoxin level is not a test point according to the applicable pharmacopoeia monographs (e.g. Ph.Eur., USP) for Purified Water. However, if PW is used as feed water for distillation and pure steam generators for the .
The newest United States Pharmacopeia (USP) Chapter <1085> Guidelines on Endotoxins Testing fills in the regulatory gaps that have resulted following the withdrawal of the 1987 FDA Guideline and implementation of its replacement Q&A. Below are answers to some of the most frequently asked questions pertaining to USP Chapter <1085>.• If the dose is 10 mg/kg/hr, the endotoxin limit is (5 EU/kg/hr) ÷ (10 mg/kg/hr) = 0.5 EU/mg • If the dose is 100mg/kg/hr, the endotoxin limit is (5 EU/kg/hr) ÷ (100 mg/kg/hr) = 0.05 EU/mg 3. There can be many endotoxin limits for one product depending on what the PD group predicts or what the fi nal package insert says aboutThe <85> Bacterial Endotoxins Test General Chapter was incorporated into and became official with the Second Supplement to USP 35–NF 30. Should you have any questions about this General Chapter, please contact Rahdakrishna Tirumalai (301-816-8339 or [email protected] ). For any questions about the PDG and its processes, please see the .
Ethide Laboratories. Laboratory Testing Services for Medical Devices in Rhode Island.
Accessing USP–NF Online To access details about the standards below and other relevant information, you must be signed in to USP–NF Online. If you are a scientist, developer or manufacturer working on COVID-19 vaccines or treatments, and would like to request free access, complete this online request. Standard name Brief description
In addition, EP 5.1.10. states that “the capability of the process to reduce or remove bacterial endotoxins during manufacture might result in lower endotoxin limits for certain processes”. These additional requirements resulted in a complex regulatory landscape because USP and ChP have not aligned with FDA and EP yet.
an endotoxin reference standard that has been calibrated against the International Standard, for exampleendotoxin standard BRP. Endotoxin is expressed in International Units (IU). The equivalence in IU of the International Standard is stated by the World Health Organisation. NOTE: One International Unit (IU) of endotoxin is equal to

Liquid medical devices such as dialysate. Implantable medical devices such as heart valves and vascular grafts, and other medical devices with a nonpyrogenic claim that may come into contact with blood or cerebrospinal fluid. Gels with a nonpyrogenic claim including demineralized bone matrices and drug delivery systems.
Endotoxin Unit (EU) is a unit of biological activity of the USP Reference Endotoxin Standard. Summary This document describes a uniquely simple system for assuring that compounded sterile products are free of endotoxin (pyrogen) within limits set by the Pharmacopeia (USP) or consistent with current scientific opinion.
usp endotoxin limits|endotoxin limits for parenteral drugs
PH0 · usp endotoxin reference standard
PH1 · usp 161 endotoxin
PH2 · how to calculate endotoxin limit
PH3 · fda guidance pyrogen and endotoxin testing
PH4 · fda endotoxin guidance
PH5 · endotoxin limits for parenteral drugs
PH6 · endotoxin limit for injectable products
PH7 · bacterial endotoxin test procedure
PH8 · Iba pa